Products-and-Services
Products-and-Services
State-Of-The Art Technology
The manufacturing plants have cGMP/WHO-GMP approval to manufacture pharmaceutical products for Domestic as well as the International market enabling us to contract manufacture and market a diverse range of products to meet export commitments as well as to continuously add new products to our range every year. Our products meet all basic pharmacopoeia standards including USP, BP & EP, and are certified for exports by neutral laboratories. The strength of our company lies in the comprehensive know-how of our supporting manufacturers covering every area of the manufacturing technology of API’s, Intermediates, Pellets & Granules in read-to-fill form and our own prestigious brands of Pharmaceutical Formulations in different dosage forms as well as in the availability of all the support services.
Quality & Service
Atlanta Associates practices a distinct business philosophy on the principles of prompt, ethical service coupled with integrity, competence, dedication and reliability. This is achieved through a sophisticated quality control network and by continuously upgrading manufacturing process and practices to the highest possible level, ensuring both consistently high product quality and strong customer confidence in our products.

Research & Development
Innovative solutions for unmet medical needs
Patent rules are being strengthened globally and there is urgent need of development of non-infringing processes of bulk drugs to capture global market. Atlanta Associates are associated with Global researchers, jointly set up by doctors, professors, advanced engineers & senior technicians and managers from enterprises, who combine to constitute a well-trained team of experienced technology developers and engineering technicians and hence are able to undertake contract manufacturing of Pharmaceutical Intermediates & Merchant export of various API's.
The supporting manufacturers of Atlanta Associates have excellent researchers with capabilities in both hardware and software developing and standardizing analytical techniques for all its products. Increased investment in Research & Development program enables our supporting manufacturers to manufacture products of the highest quality. Today these researchers are dedicated to discovering and developing innovative healthcare products.
Yes, at Atlanta Associates, our young, dedicated and hard working team would indeed be delighted to serve you. No matter how difficult the task may be, our team makes it easy.

Quality Control
Innovative companies are rapidly creating an exhaustive quality system and our supporting manufacturers are no exception having well equipped and well managed Quality Control Department. The requisite quality systems conforming to National and International regulations have been implemented across all operations to ensure qualitative output at each step being provided with requisite infrastructure and expertise to ensure proper regulatory approvals. Well defined process controls and documentation procedures are the key to achieving quality documentation and to ensure qualitative output, meeting all the pre-determined specifications consistently. All products manufactured are as per the regulations and guidelines of the ICH and World Health Organization's Good Manufacturing Practice (WHO-GMP).
The quality policy not only extends through in-house operations but also extends to vendors who are our suppliers for Active Product Ingredients and excipients. All the processes are backed by a sophisticated Quality Assurance System ensuring Quality product at each and every step - from acquiring of Raw materials to the Finished Formulations. Quality Control, which forms a key unit of a Quality Assurance System, is well equipped with most sophisticated, ultra-modern and state-of-the-art instruments.
Quality Assurance
Building quality into each product
Quality forms the fulcrum of our business practice and philosopy. Our supporting manufacturers are committed to providing high-quality products and services, and implement a quality management system in compliance with regulatory requirements throughout the life cycle of the product, so as to meet the customer demands.
Quality Policy
In close cooperation with our supporting manufacturers we understand our customer’s requirements, and accordingly we ensure systems and procedures are developed. We always put customers at the centre of our attention.
Our supporting manufacturers implement the quality system throughout the life cycle of each product, and we comply with all regulatory requirements and laws.
Our supporting manufacturers continuously search for and correct system deficiencies, and make improvements in pursuit of quality excellence.
Quality is Build into Product
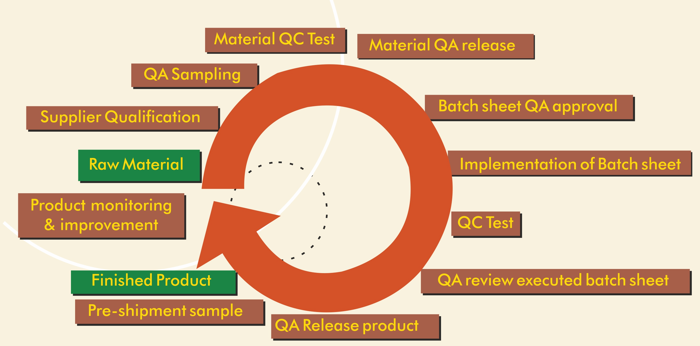
Quality assurance is dynamically structured in line with international markets. Our processes include testing of inputs, development and adoption of more accurate methord of analysis, process validation of manufacturing, following the Customer’s product specification, packing requirements, etc. Analytical support for lab and production ranges from the release of raw materials to in-process control. This includes the development of chromatographic methods as well as structural determination of impurities and by-products for release testing. The supporting manufacturers of Atlanta Associates manufacturing units is equipped with latest high precision instruments, having spared no efforts to raise its quality standards to international levels and have acquired and developed the requisite skills and expertise to strengthen in-house skills through extensive training and multiple technical audits.
Quality Assurance Department has advanced quality control equipment and an excellent team of professionals having good understanding of concepts of quality systems ensuring that products are manufactured responsibly and in accordance with WHO-GMP standards, forming a strict quality assurance system.
Activities and Responsibilities:

Comprehensive thorough quality processes
- Release of qualification & validation protocols.
- Release of documents eg. Specifications, Master Batch Records, SOP’s.
- Batch review and release, archiving.
- Release of Batch Records
- Assessment and approval of their change control, deviations from analytical procedures and process deviation control, investigation reports about raw material and internal complaints.
- Approval of validation protocols.
- Scrutiny of the complete batch documentation.
- Training.
- Internal audits compliance.
- Supplier qualification and supplier audits.
- Claims, recall, etc.
Records of manufacturing are properly documented
All the analytical test procedures and manufacturing procedures are well documented and revision is undertaken as per specified protocol. Analytical methods are validated to give the reproducible results. Stability study as per stability protocol is considered to be very important area of Quality Assurance.
Utilities
There are many utilities attached with manufacturing of each section at all the units which are assigned to Atlanta Associates, approved by FDA, for contract manufacturing under loan license which are provided with the same. The common utilities are washing of Bottles, Vials, Ampoules, Inspection of filled Vials & Ampoules and Bottles, Rubber Bungs washing & autoclaving, Autoclave of Vials & Ampoules, Tablet Printing, Ceramic printing on Vials & Ampoules, etc. Bottle washing from inside & outside is done with liquid detergent, portable water and DM water. The machine has also a provision of air to dry the bottles from inside. The washed bottles are dried up in dryer and are then sent for filling.
