Regulatory Affairs

Regulatory Affairs
Atlanta Associates close association with strategic manufacturers enables us to respond quickly and reliably to quality assurance issues and technical service requests – and assist in all aspects concerning regulatory submission. Our experience, knowledge, and strong working relationships with regulatory agencies help us get speedy approvals to bring customers to market faster. We deliver a comprehensive set of services to all customers with the support of our manufacturing partners for formulation and process development, clinical sample supplies, scale up, development and validation of analytical methods, stability studies (as per ICH guidelines), and also dependable regulatory dossier support. All these woud ensure that your products reach the market in the shortest possible time with the highest level of quality. This will help each our customers to fulfill their immediate needs and expectations.
Atlanta Associates has the strategic and technical expertise in documentation related to batch production, quality, and regulatory documents. The regulatory documents include DMF, CEP, Technical Package, and Registration Dossiers. All these documents are strictly prepared and submitted as per CTD & e-CTD formats & the latest guidelines of health authorities of relevant countries. Whereas all quality-related documents are prepared according to WHO-GMP guidelines.
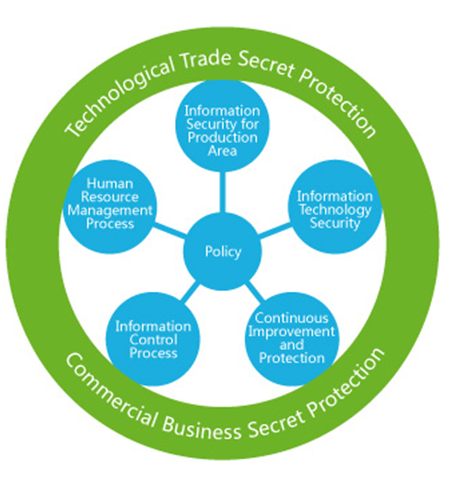
Intellectual Property Protection (IPP) and Trade Secret Protection (TSP)
Protecting intellectual property rights is one of our core principles, one of which is always kept in mind as it is of important strategic significance to the survival and future development of both our customers and ourselves. To protect our clients' IP, our supporting manufacturers have integrated complete and strict procedures into their daily operations aimed at protecting and maintaining the storage of data and other records. Atlanta Associates have undertaken professional services of in-house legal experts specialized in the Intellectual Property Protection (IPP) and Trade Secret Protection (TSP) laws.
